Enzymes laboratory experiment report by Steven Trujillo
Question
How do abiotic or biotic factors influence the rates of enzymatic reactions (chemical reactions that are assisted by enzymes)?
Background
Enzymes speed up chemical reactions by lowering activation energy (that is, the energy
needed for a reaction to begin). In every chemical reaction, the starting materials (the
substrate(s) in the case of enzymes) can take many different paths to forming products.
For each path, there is an intermediate or transitional product between reactants and
final products. The energy needed to start a reaction is the energy required to form that
transitional product. Enzymes make it easier for substrates to reach that transitional
state. The easier it is to reach that state, the less energy the reaction needs.
Enzymes are biological catalysts. They are large protein molecules, folded so that they
have very specifically shaped substrate binding sites. These binding sites make substrates
go into the transition state. To catalyze the reaction, several regions of the binding site
must be precisely positioned around the substrate molecules. Any change in the shape of
the overall folded enzyme molecule can change the shape of the binding site.
The optimum reaction conditions are different for each enzyme. The correct
environmental conditions, proper substrates, and, often, particular cofactors associated
with an enzyme are needed. In some instances, the optimum conditions can be deduced
fairly accurately based on the following:
-
The organism from which the enzyme is derived
-
The part of the organism in which the enzyme functions
-
The environmental conditions in which that organism lives
Take the example of lactase, an enzyme that catabolizes (breaks down) the disaccharide sugar lactose into the two monosaccharides, glucose and galactose. In humans, lactase is found mostly in the small intestine, where the pH is around 7. It would be reasonable to hypothesize that human lactase is optimally active at pH 7 and at 37°C (normal human core body temperature in degrees celsius). Free-living decomposer fungi in soil also produce lactase. However, soil pH usually is between 5 and 6.5. As could be predicted, the purified enzyme from a common soil fungus has a pH optimum of 5.5. The main enzyme for this lab, peroxidase, is found in many different forms, with optimum pHs ranging from 4 to 11 depending on the source and optimum temperatures varying from 10 to 70°C.
The organism from which the enzyme is derived
The part of the organism in which the enzyme functions
The environmental conditions in which that organism lives
Purpose
In this experiment you will investigate the effect of environmental factors on the enzyme hydrogen peroxidase. This enzyme is found in all aerobic (using oxygen) cells and functions to decompose hydrogen peroxide into O2(g) and H2O. The specific environmental factors you will test (as a class) are temperature, pH, substrate concentration, and enzyme concentration. Your team will select one of these factors (variables) to test and report on.
Materials
-
-
Digital balance (scale)
-
Mortar and pestle
-
Distilled water
-
3 100-liter glass or plastic beakers
-
1 mL or 5 mL syringe
-
Hydrogen peroxide
-
1 Paper towel square (for filtration)
-
Glass test tubes
-
Test tube rack or holder
-
Small plastic ruler
-
Safety glasses
Depending on which environmental factor you choose to investigate, some of the following items will be needed for your experiment:
-
Ice
-
Large plastic beaker (for ice bath)
-
Hot water
-
Large plastic beaker (for hot water bath)
-
Thermometet
Digital balance (scale)
Mortar and pestle
Distilled water
3 100-liter glass or plastic beakers
1 mL or 5 mL syringe
Hydrogen peroxide
1 Paper towel square (for filtration)
Glass test tubes
Test tube rack or holder
Small plastic ruler
Safety glasses
Ice
Large plastic beaker (for ice bath)
Hot water
Large plastic beaker (for hot water bath)
Thermometet
Procedure
First we are going to grab grass and mash it up adding 10 mL of water. Second we are going to grab a tray of ice water or warm water. Third we are going to take the nine test tubes and put 2 in cold water, 3 in room temperature, and 3 in warm water. Lastly we measure the height of the foam and compare the data, to see if our hypothesis is correct.
This part will be determined by the students conducting the experiment. For an overview of the general scientific experimentation and research process, see the flow diagram below. Use the worksheet that follows to write out in detail the hypothesis you are seeking to test, the materials you will use, and the steps you will follow to conduct your experiment.
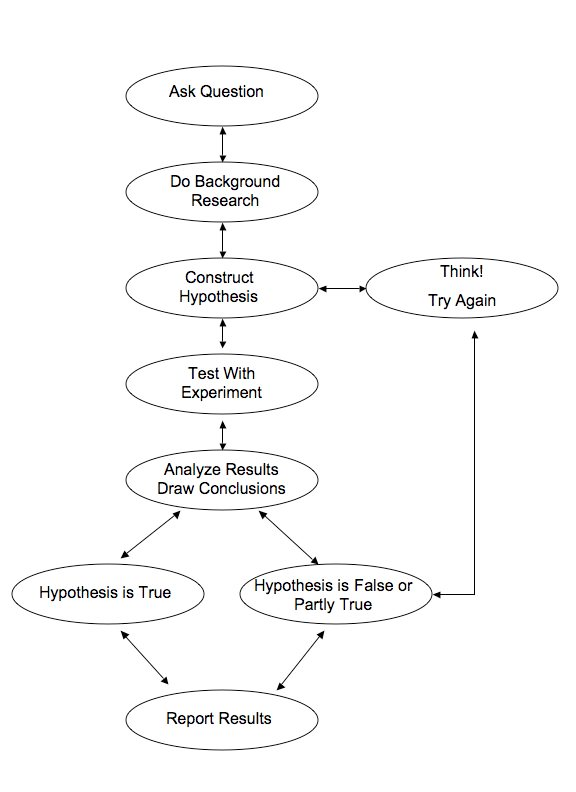
Enzyme Lab Worksheet
Hypothesis: If enzymes dont function well under could temperatures, then the reaction should be greater when we up the temperature.
Independent Variable:
Heat of water
Dependent Variable:
Height of foam
Controlled Variables:
Ammount of water to enzyme ratio 1/1
Justification of hypothesis:
We choose to change the heat of the water because we learned that could slow the process and that heat speeds it up,but if there is too much heat it will mess it up.
Why did you choose this as your hypothesis?
Materials (Your Team’s Experiment):
-
Handful of grass
-
Water
-
Water heater
-
Ice
-
Graduated cylinder
-
thermometer
-
Hydrogen peroxide
-
Glass test tubes
-
Test tube rack
-
Paper towels
-
Syringe
-
Small plastic ruler
Handful of grass
Water
Water heater
Ice
Graduated cylinder
thermometer
Hydrogen peroxide
Glass test tubes
Test tube rack
Paper towels
Syringe
Small plastic ruler
Procedure:
First we collected the grass and mashed it up to obtain the extract. Second we put the extract milliliter in the test tubes and placed 1 milliliter of peroxide into the test tubes and then we flicked them and started the timer for two minutes and waited. We placed them in the temperature we wanted for the time. After the time we took them out and recorded the height of the foam. Lastly we compared the data to see if our hypothesis is correct.
Summary:
For our lab we had our hypothesis proven correct. We ran a test run in room temperature to know what to expect. The data we got was warm water speeds up enzyme reactions, unlike cold water that slows them down.
Detailed Steps:
-
Measure out 10 grams of grass
-
Measure 10 mL of water
-
Pour water and grass into mortar and pestle
-
Grind grass and water thoroughly
-
Dump mixture into paper towel
-
Squeeze mixture out into beaker
-
Pour hydrogen peroxide into separate beaker
-
Mix the two in temperature wanted
-
Wait two minutes
-
Pull out and record height of foam
Measure out 10 grams of grass
Measure 10 mL of water
Pour water and grass into mortar and pestle
Grind grass and water thoroughly
Dump mixture into paper towel
Squeeze mixture out into beaker
Pour hydrogen peroxide into separate beaker
Mix the two in temperature wanted
Wait two minutes
Pull out and record height of foam
Comments
Post a Comment